
As a result, when stated in daltons, the numeric value of the atomic mass is roughly equal to the mass number. Protons and neutrons in the nucleus make up nearly all of an atom’s mass, with electrons and nuclear binding energy playing a minor contribution. Although the kilogramme is the SI unit of mass, the atomic mass is sometimes stated in the non-SI unit dalton, which is defined as 1/12 of the mass of a single carbon-12 atom at rest. The mass of an atom is its atomic mass.The number of electrons in an uncharged atom is also equal to the atomic number. It is the same as the charge number of the nucleus. Sodium is a chemical element of the periodic table with chemical symbol Na and atomic number 11 with an atomic weight of 22.9898 u and is classed as a. The number of protons in the nucleus of each atom of a chemical element is known as its atomic number.The only way to identify a chemical element is by its atomic number.We know that because sodium’s atomic mass is 23, we can write it as 23 g/mol. The atomic number of sodium is 11, while the number of neutrons in its nucleus is 12.Īs a result, 1 mole of sodium equals 23 grams. In the periodic table, it is the eleventh element. It is the first element of the third periodic period in the periodic table.
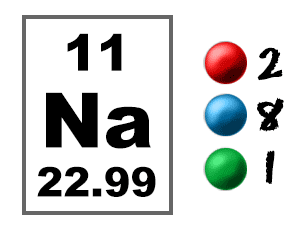
Sodium is a metal that belongs to the alkali family. Which of the following statements is correct regarding number of protons, electrons and. The following formula was used: Number of neutrons + Number of protons An atom of sodium has an atomic number of 11 and a mass number of 23. Answer: (b)Įxplanation: The atomic mass of an element can be calculated using the element’s atomic number and the number of neutrons present.
What is the Atomic Mass of Sodium? – Chemistry Q&A (a).


 0 kommentar(er)
0 kommentar(er)
